Oozing with Life, and Maybe Iron
The Palmer has an ample supply of Dutch hot chocolate mix to warm people as they come in from the wind, spray, and snow on deck. Today I fixed my cup as normal, but I walked away from the galley without a spoon.
As I sloshed and swirled my cup, hoping the hot chocolate powder would mix with the hot water, I realized I was facing the same problem that phytoplankton face in the Ross Sea. At the bottom of my cup were a whole bunch of particles that I wanted, while at the top there was only clear water.
If I could get just some of the powder up to the top, the water would be sweet and chocolatey, and I’d be happy. But even though I was adding a considerable amount of energy in the form of swirling, I still had to go back and get a spoon. If all that work couldn’t mix the chocolate just a few inches in my cup, how does mixing happen at sea, where nutrients have to move up through thousands of feet of water?
This is one question that Dr. Phoebe Lam has spent much of this voyage thinking about—and it has prompted her to start looking at what lies on the bottom of the Ross Sea in more detail. We’ve been looking over her shoulder. Read on through the slideshow to see what she has found:
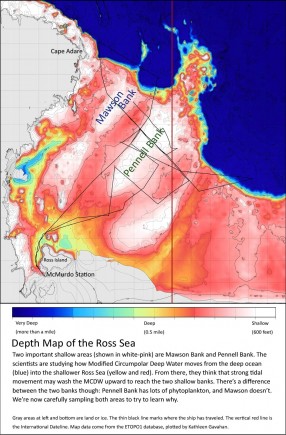
- As results came back showing that MCDW contains surprisingly little iron (see yesterday’s post), Dr. Lam began wondering if iron could be washing upward in the form of particles from the floor of the Ross Sea. Talking with Bruce Huber, Dr. Josh Kohut, and other scientists on the ship, she realized that strong tidal currents in the area might provide enough energy to carry sediment up toward the surface (see the map after the slideshow).
- Dr. Lam uses this device, called a Smith-Mac grab, to take a bite out of the seafloor. It’s about 2.5 feet high, an industrial concoction of springs, counterweights, struts, safety releases, and lead weights. When it strikes the bottom, two curved jaws snap shut to dig in and capture whatever the seafloor is made of.
- When the mud grabber comes back on deck, Dr. Lam has a hefty sample of mud to work with. Dr. Lam started this expedition interested in sediment for a different analysis involving trace amounts of an element called neodymium. For that, she brought along a smaller mud sampler, but it didn’t work properly. It was a happy accident. When she started using the Smith-Mac she realized it carried enough mud that she could analyze iron content of the sediment and its water. Those measurements will help the scientists understand one more way iron might be reaching the surface waters.
- Clad in purple nitrile gloves to keep from contaminating her sample, Dr. Lam extracts samples of slick green mud from the Smith-Mac. Next she’ll spin the samples in a centrifuge to separate the wet particles from the water all around them. Both of these could carry iron if they get picked up by currents. She gives the water to Dr. Chris Measures’s team and saves the particles to analyze when she gets home.
- This mud has a great name: diatomaceous ooze. It’s sticky, cold, and green, and it feels like thick grease if you squeeze it and let it squish out between your fingers. It’s made largely of the bodies of dead diatoms—the residue of phytoplankton blooms from years and centuries past. As Dr. Lam pours the leftovers back into the sea, an unfamiliar, terrestrial scent mixes with the chilly salt air.
- Though it’s not essential for Dr. Lam’s sediment chemistry work, we combed through the mud to see what was living in it. The water at the seafloor is about 28°F (it would be frozen if it weren’t so salty), it’s pitch dark, and the pressure is 40 times what we feel up here on the Palmer. So pretty much anything we find is going to be interesting to look at. This is a tubeworm. It lives inside its pale tube and pushes out feathery arms to collect food that floats by on currents.
- Dr. Scott Fay pointed out a few flat, grayish strips of what looked like fossilized snakeskin. They’re colonies of hundreds of little animals called bryozoans. Like corals and a lot of other sea life, they make hard shells out of calcium carbonate for protection. The bryozoans’ shells are long hexagons all attached to each other. Each bryozoan has a hole through which it extends its own type of feathery appendage to filter food particles.
- Almost every hard thing that Chris and I pulled out of the mud we wanted to call a coral, but thankfully a variety of biologists were around to tell us we were wrong. However, everyone we talked to agreed that this brittle, antler-shaped relic was indeed a coral… probably. Even though our ship is packed with experts in many fields, we don’t have anyone who specializes on life you can see without a microscope. So if you recognize this, please let us know.
- Dr. Lam used a large sieve to strain one of the mud samples she collected. Among many dark volcanic pebbles she found this collection of shells, or skeletons, of sea life. Do those rough strips look like snakeskin to you? Those are the bryozoans you saw up close two slides ago. The two curved tubes that look like miniature horns are a kind of mollusk, Dr. Fay told us. Just in front of them is a small, smooth, egg shape. That’s a creature called a foraminifer, a single-celled creature that lives in the top layer of sediment. For his Ph.D., Dr. Fay studied the ways forams and algae live together, and he was impressed with this one’s size. ‘I didn’t know these things got so big. I’m really blown away here,’ he said, only half joking.
- After Chris and I finished looking through Dr. Lam’s tray of rocks, Dr. Fay put them under the microscope. He grabbed a fine-pointed paintbrush, licked the tip, and started picking out these tiny foram shells. He identified Nodosinum, the long, scepter-shaped one at top left, and Textelaria, the little one at center right that looks like a leaf. Great numbers of forams can build up on the seafloor, fossilize, and turn into rock. As a graduate student, Dr. Fay visited the pyramids of Egypt, where the individual stones inspired him at least as much as the architecture. ‘The rocks the pyramids are made of are just chock-a-block with forams,’ he said. ‘The Romans thought they were coins. But they’re made out of solid, giant forams.’
For the rest of our expedition, the Palmer is going to crisscross two shallow parts of the Ross Sea: Mawson Bank and Pennell Bank. Dr. Lam will be sampling the seafloor as we cross, and Dr. Kohut’s and Bruce Huber’s teams will measure the currents that flow along the seafloor. Here’s a map of the area to help you keep straight the parts of the Ross Sea we’re studying:
If the Ross Sea is getting mixed like a giant cup of hot chocolate, how is it happening? Dr. Lam’s analyses will tell us about the particles on the seafloor: how much iron is there, and whether particles of it might be reaching the phytoplankton.
To move that iron up to the surface waters will take a lot of energy. Dr. Kohut’s and Bruce’s measurements will help decide if the bottom currents are providing enough. And the terrain of the seafloor, as shown on the map, might be acting like that spoon I needed today—a hard surface that deflects the swirling currents upwards. That’s why the scientists are focusing on these two shallow banks and their sloping sides.
Many thanks to Dr. Angelicque White and Dr. Scott Fay for help with the seafloor creatures, and to Kathleen Gavahan for plotting the map.
Read more in the following posts:
- Checking in With Our Hypotheses (Feb 7)


 February 8, 2011
February 8, 2011 

















12 Responses to “Oozing with Life, and Maybe Iron”