Silicon
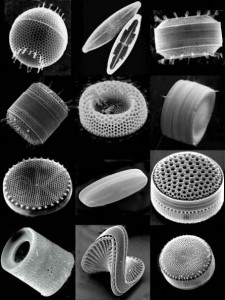 Silicon (Si) is element 14 in the periodic table. In the earth, it is one of the most abundant elements, linking with four oxygen atoms to form the silicate tetrahedron, the building block of most minerals found in rocks. In the ocean, it has a very important role. It is slightly soluble (able to be dissolved), in the form of silicic acid (H₄SiO₄). It is assimilated (used) by diatoms. These are small phytoplankton, who grow tests (a fancy word for shell-like structures) made of SiO₂. Diatoms can grow quickly and to fairly large sizes, and when they die, or are eaten, the SiO₂ in the tests helps the remains to sink into the deep ocean, carrying organic carbon with them. Diatoms are the most important player in the biological carbon pump that helps transport CO₂(dissolved in the ocean from the atmosphere) to sink from the surface ocean to the deep ocean.
Silicon (Si) is element 14 in the periodic table. In the earth, it is one of the most abundant elements, linking with four oxygen atoms to form the silicate tetrahedron, the building block of most minerals found in rocks. In the ocean, it has a very important role. It is slightly soluble (able to be dissolved), in the form of silicic acid (H₄SiO₄). It is assimilated (used) by diatoms. These are small phytoplankton, who grow tests (a fancy word for shell-like structures) made of SiO₂. Diatoms can grow quickly and to fairly large sizes, and when they die, or are eaten, the SiO₂ in the tests helps the remains to sink into the deep ocean, carrying organic carbon with them. Diatoms are the most important player in the biological carbon pump that helps transport CO₂(dissolved in the ocean from the atmosphere) to sink from the surface ocean to the deep ocean.
On this cruise, one thing we are studying is the fate of the diatom remains. As they sink, how fast does the Si dissolve, recycling Si for re-use? How much of the dissolution occurs once these tests hit the sea floor? Is a large fraction of Si buried and not recycled? The behavior of the silicon cycle is very important in regulating the ocean carbon cycle, as Si availability limits diatom growth.
Written by: Dr. Doug Hammond


Recent Comments